New Therapeutic Targets to Fight Type 2 Diabetes
One of the aspects that most confounds those affected by type 2 diabetes mellitus is the elevated fasting glucose levels they present. This situation is explained by the fact that in these insulin-resistant patients, glucose production by the liver is triggered, a process still full of questions for the scientific community.
Now, a review article published in the journal Trends in Endocrinology & Metabolism presents an integrative view of the most significant advances in understanding this process and helping to identify new pharmacological targets in the fight against type 2 diabetes mellitus, considered by the World Health Organisation (WHO) as one of the pandemics of the 21st century.
The research is led by Professor Manuel Vázquez-Carrera, coordinator of the Pharmacological Targets in Inflammation and Metabolic Diseases research group and a researcher at the Faculty of Pharmacy and Food Sciences at the UB, the Institute of Biomedicine of the UB (IBUB), and the Centre for Biomedical Research in Diabetes and Associated Metabolic Diseases Network (CIBERDEM). The participation of experts Emma Barroso, Javier Jurado-Aguilar, and Xavier Palomer (UB-IUB-IRJSJD-CIBERDEM) and Professor Walter Wahli from the University of Lausanne (Switzerland) is also notable.
Therapeutic targets to fight the disease
Type 2 diabetes mellitus is an increasingly common chronic disease that generates high levels of circulating glucose - the cellular energy fuel - due to a poor insulin response in the body. It can cause severe damage to various organs and is estimated to be underdiagnosed in a large percentage of the affected population worldwide.
In patients, the glucose synthesis pathway in the liver (gluconeogenesis) is hyperactivated, a process that can be controlled by drugs such as metformin.
"In recent years, new factors involved in the control of hepatic gluconeogenesis have been identified. For example, a study by our group revealed that the factor GDF15 reduces the levels of proteins involved in hepatic gluconeogenesis," explains Professor Manuel Vázquez-Carrera, coordinator of the Pharmacological Targets in Inflammation and Metabolic Diseases research group.
To advance in the fight against this condition, it will also be necessary to delve deeper into the study of pathways such as that of the TGF-β factor, which is involved in the progression of metabolic dysfunction-associated fatty liver disease (MASLD), a very prevalent pathology that often coexists with type 2 diabetes mellitus. "TGF-β plays a very important role in the progression of liver fibrosis and has become one of the most important factors that can contribute to the increase in hepatic gluconeogenesis and, therefore, type 2 diabetes mellitus. Therefore, studying the involvement of the TGF-β pathway in the regulation of hepatic gluconeogenesis could help achieve better glycaemic control," emphasises Vázquez-Carrera.
However, acting on a single factor to improve the regulation of gluconeogenesis does not seem to be a sufficient therapeutic strategy to adequately control the disease. "It would be important to design combined therapies that could consider the different factors involved to improve the approach to type 2 diabetes mellitus," the expert indicates.
"Nowadays, there are several molecules - TGF-β, TOX3, TOX4, etc. - that could be considered therapeutic targets to design future strategies and improve patients' well-being. Their efficacy and safety will determine their therapeutic success. We must not lose sight of the fact that controlling the overactivation of hepatic gluconeogenesis in type 2 diabetes mellitus has an additional difficulty: it is a key pathway for glucose availability during fasting, finely modulated by numerous factors, making regulation difficult."
Interestingly, other factors involved in the control of gluconeogenesis have also been identified in patients hospitalised with COVID-19 who showed high glucose levels. "Hyperglycaemia was very prevalent in patients hospitalised with COVID-19, which seems to be related to the ability of SARS-CoV-2 to induce the activity of proteins involved in hepatic gluconeogenesis," the expert points out.
Metformin: the unknowns of the most prescribed drug
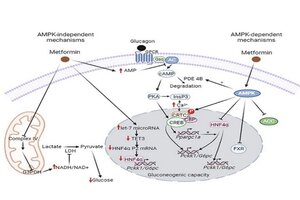
Not all the mechanisms of action of metformin, the most prescribed drug for the treatment of type 2 diabetes, which reduces hepatic gluconeogenesis, are yet known. It has now been discovered that this drug reduces gluconeogenesis by inhibiting complex IV of the mitochondrial electron transport chain. This is an independent mechanism from the classic effects previously known through the activation of the AMPK protein, a cellular energy metabolism sensor.
"Inhibition of mitochondrial complex IV activity by metformin - not complex I as previously believed - reduces the availability of substrates necessary for hepatic glucose synthesis," says Vázquez-Carrera.
Moreover, metformin can also reduce gluconeogenesis through its effects on the intestine, causing changes that ultimately attenuate hepatic glucose production. "Thus, metformin increases glucose uptake and utilisation in the intestine, generating metabolites capable of inhibiting gluconeogenesis when they reach the liver via the portal vein. Finally, metformin also stimulates the secretion of GLP-1 in the intestine, a peptide that inhibits hepatic gluconeogenesis and contributes to its antidiabetic effect."
Currently, the team led by Vázquez-Carrera continues their research to decipher the mechanisms by which GDF15 might regulate hepatic gluconeogenesis.
"In parallel, we aim to design new molecules that increase circulating levels of GDF15. If we have potent inducers of GDF15, it could improve glycaemia in those affected by type 2 diabetes mellitus by reducing hepatic gluconeogenesis, but also through other actions of this cytokine," concludes the researcher.
Reference article
Barroso E, Jurado-Aguilar J, Wahli W, Palomer X, Vázquez-Carrera M. Increased hepatic gluconeogenesis and type 2 diabetes mellitus. Trends Endocrinol Metab. 2024 May 29 (24)00124-3. doi: 10.1016/j.tem.2024.05.006. Epub ahead of print. PMID: 38816269.
Designing combined therapies based on the different factors involved in the control of glucose synthesis in the liver could be a more effective strategy for addressing the treatment of this disease in patients.
